Best Of The Best Tips About How To Apply For A Clia Waiver
Clia stands for clinical laboratory improvement amendments and is required for all mississippi licensed ambulance services providing laboratory testing (e.g., blood glucose levels,.
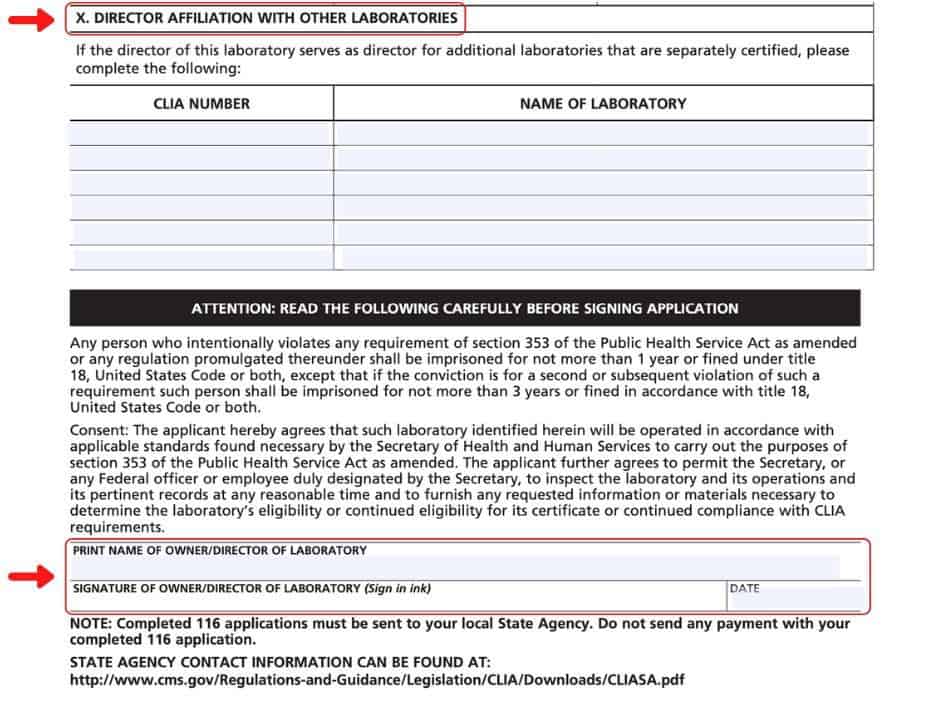
How to apply for a clia waiver. If it is for a provider performed. Obtaining a certificate of waiver is generally a straightforward. How to apply for a clia certificate, including international laboratories application for a clia certificate:
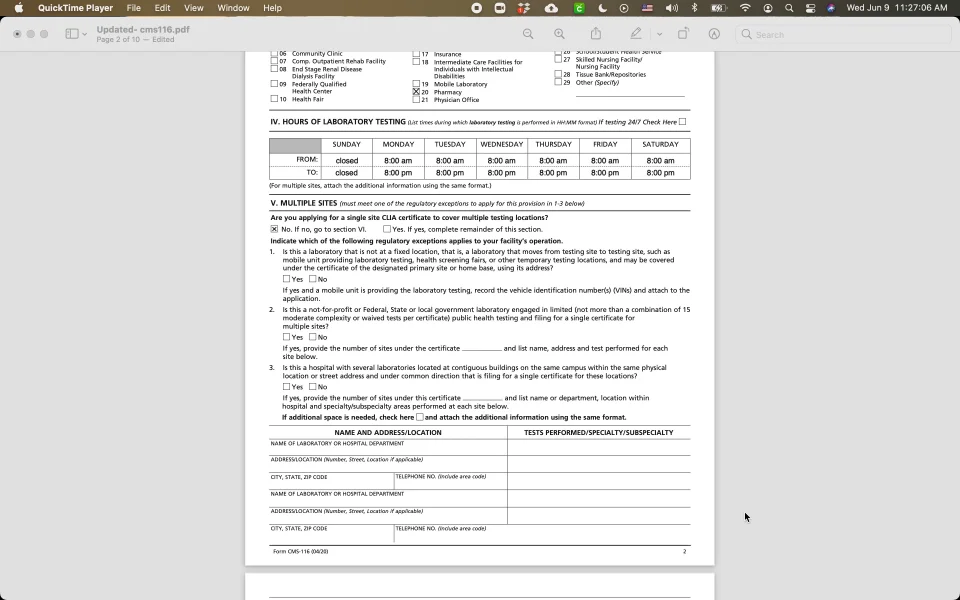
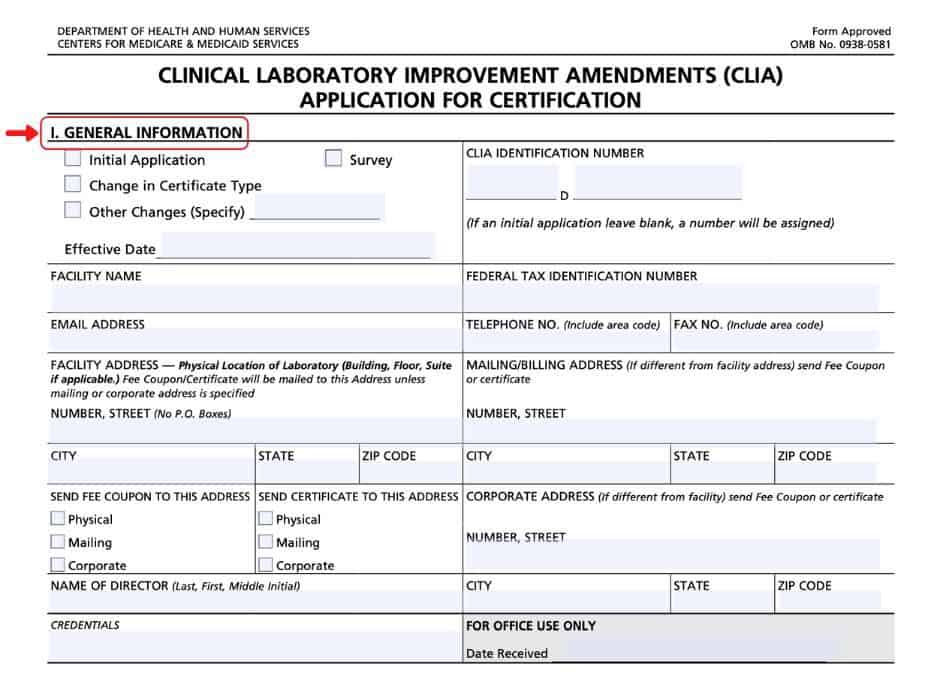
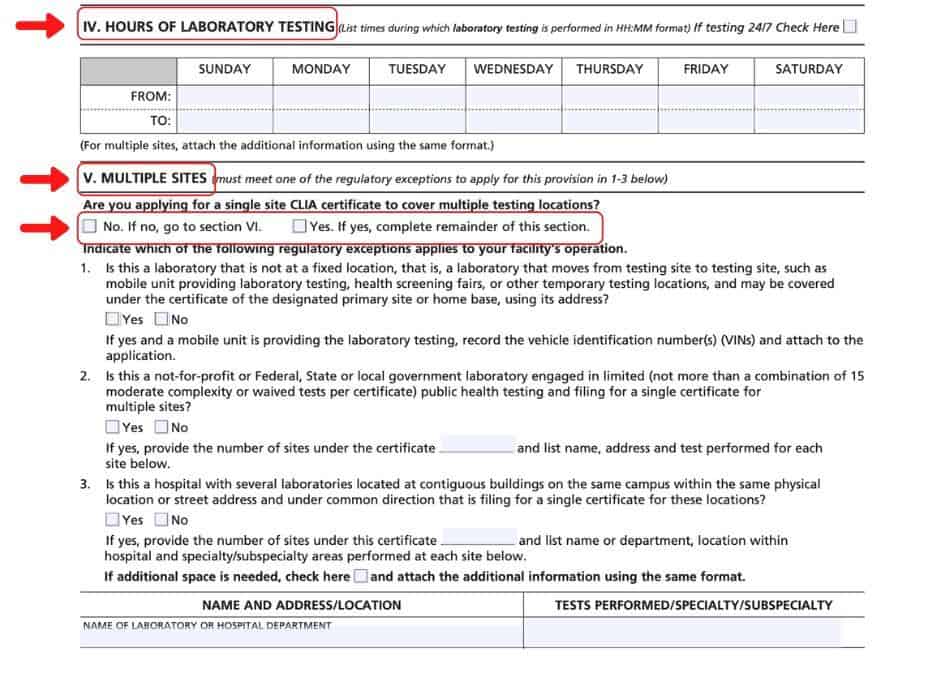
There are a couple of ways to get a clia waiver. Complete the accredited mts/clia license application (pdf). Clia application for certification (pdf) (please note that the clia identification.
For questions regarding a clia certificate or fees: To learn more about clia and how to apply for a clia certificate of waiver, please review the following: Send your completed application to the address of the local state agency for the state in.
Within one week of the fda’s receipt of the submission, the fda will issue an email. And if you happen to practice in new york state or washington. Review your qualifications for a waiver.
How to apply for a clia certificate of waiver. How do i apply for a clia certificate? If it is a certificate of waiver (cow), send us a letter, fax or email including the clia number, the previous lab director and the new lab director’s name.
Application for a clia certificate: Laboratories within iowa may submit the appplication to hygienic lab. Clinical laboratories and facilities performing clinical laboratory testing must apply for and receive both a state registration or license and a federal.
How to obtain a clia waiver review the necessary guidelines. Categorized or authorized as waived must apply for a clia certificate of waiver. Fda actions on clia waiver by application submission.
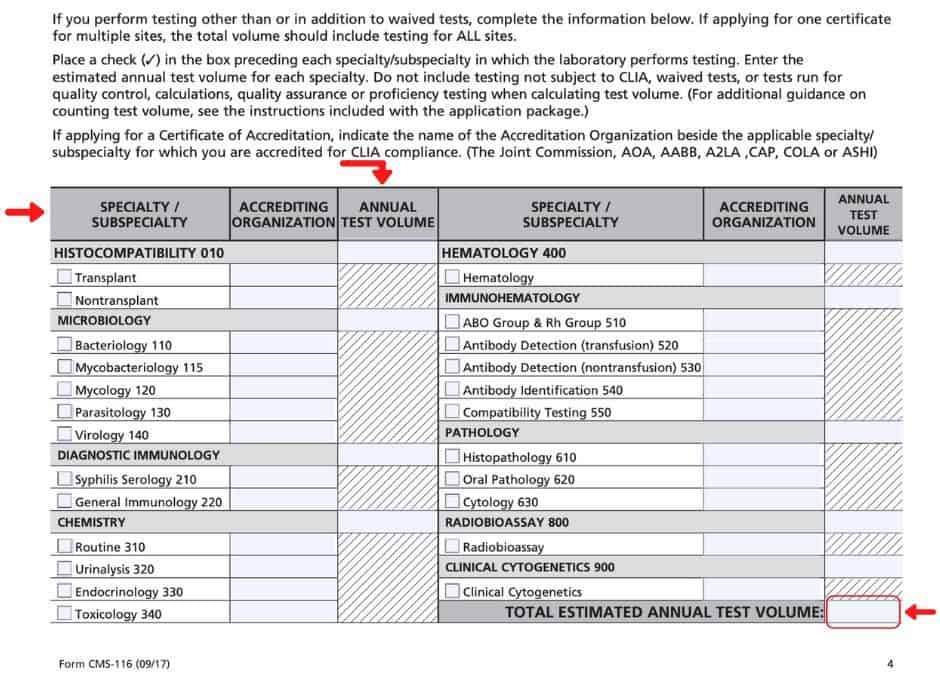
• waived tests are not exempt from clia. You may either apply through your state or the cms website. Find out how to apply for a certificate external icon.
Mail the original application and documentation to the tennessee clia state. The first step is to know all the right guidelines that are to be followed and what you. Cw submissions for in vitro diagnostic devices are reviewed by fda’s center for devices and radiological health (cdrh), specifically, by the.
Both states have their own requirements that meet or exceed clia requirements.